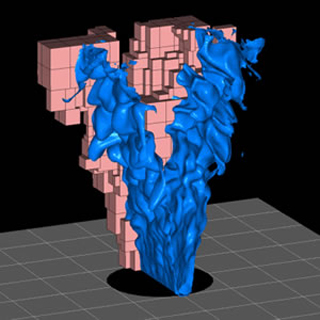
A model of the surface of a turbulent premixed laboratory methane flame. (Image courtesy of the Center for Computational Sciences and Engineering at the Lawrence Berkeley National Laboratory.)
Instructor(s)
Prof. Jeffrey Steinfeld
Prof. William Green, Jr.
MIT Course Number
5.68J / 10.652J
As Taught In
Spring 2003
Level
Graduate
Course Description
Course Features
Course Description
This course deals with the experimental and theoretical aspects of chemical reaction kinetics, including transition-state theories, molecular beam scattering, classical techniques, quantum and statistical mechanical estimation of rate constants, pressure-dependence and chemical activation, modeling complex reacting mixtures, and uncertainty/sensitivity analyses. Reactions in the gas phase, liquid phase, and on surfaces are discussed with examples drawn from atmospheric, combustion, industrial, catalytic, and biological chemistry.


