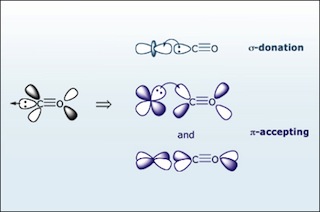
Carbon monoxide (CO) is a π accepting ligand. The bonding of CO to a metal has two components: σ bonding and π backbonding. (Image by MIT OpenCourseWare.)
Instructor(s)
Prof. Daniel Nocera
MIT Course Number
5.04
As Taught In
Fall 2008
Level
Undergraduate
Course Description
Course Features
Course Description
This course provides a systematic presentation of the chemical applications of group theory with emphasis on the formal development of the subject and its applications to the physical methods of inorganic chemical compounds. Against the backdrop of electronic structure, the electronic, vibrational, and magnetic properties of transition metal complexes are presented and their investigation by the appropriate spectroscopy described.


